India has faced many challenges in dealing with the coronavirus infection but has also achieved success. Now, along with dealing with the COVID-19 infection, there is a double challenge of making the COVID-19 vaccine available to the huge population of India. When the COVID-19 vaccine is introduced in India, it will be a record in itself.
The coronavirus vaccination will be the world’s largest vaccination campaign ever. According to FICCI, three lakh people will be required in the first phase in India to inject the vaccine which will include one lakh trained staff and two lakh volunteers. This data seems high but a large network for vaccination is already ready in India.
India is the largest vaccine manufacturer in the world, and its immunization program, such as the vaccination campaign, is so large that India will need to increase it a little more to inject the vaccine.
A person should download CO-WIN APP to register for the vaccine. However, the version that will be launched for the general public may be slightly different than those for the Health Care Workers. India has 27,000 Cold Chain Points, 76,000 Cold Chain Equipments, 55,000 Cold Chain Handlers and 700 Cold Vans.
In India in the first phase, vaccines will be given to Health Care Workers i.e. doctors, nurses and other medical staff treating coronavirus infected patients, lab experts engaged in testing and research etc. In the second phase, the vaccine will be provided to the front line workers. It will include the army, policemen, media personnel, sanitation workers. In the third phase, people over 50 years of age and sick people below 50 years will be vaccinated.
The first phase of dose delivery is planned to be completed in 7 to 10 days all over the country. The second dose will be given 28 days after the first injection.
The team of Zee Media visited the cities and villages of India to take stock of the vaccine preparations. On the basis of this report, we can say with confidence that India is only waiting for the approval of the vaccine at this time. The necessary infrastructure for the vaccine has been prepared and where there is a shortage, the need is being met.
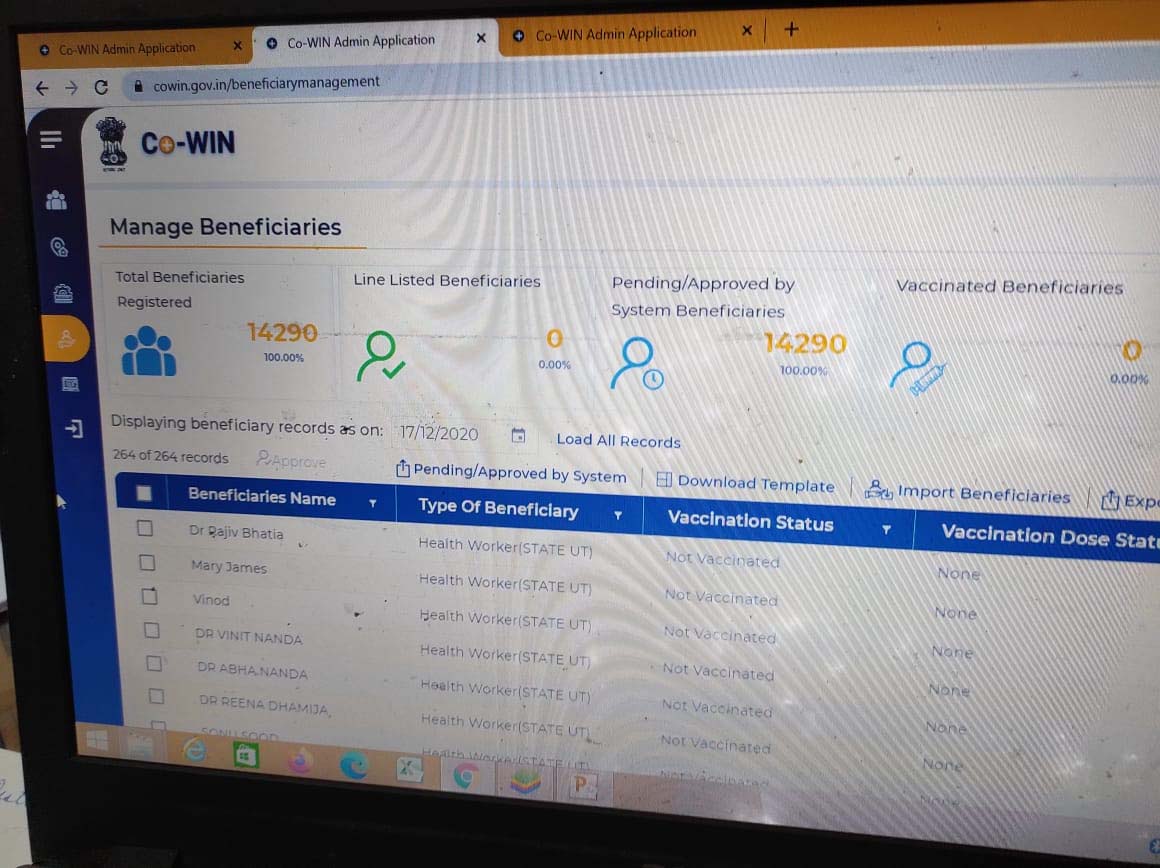
The Co-Win APP is rapidly registering the data of those who will be vaccinated in the first phase. Access to CO-WIN APP is currently available only to those who have to carry out the vaccination campaign. By looking at this report today, you can also understand how the CO-WIN APP will work.
In Uttar Pradesh’s Meerut district, files, papers or training is going on in the government hospital and all work is being done only for the coronavirus vaccine. The syringes that are being made have started to reach different cities in India rapidly. Meerut had indicated the need for six lakh syringes–the first consignment of two lakh syringes has just arrived.

Cold chain system–that is, where the vaccine needs to be stored at the required temperature. Two of the three vaccines awaiting approval in India can be easily stored here. The required minus 30 degrees for the corona vaccine is being maintained here. Yes, the required minus 70-degree storage for the Pfizer vaccine is selected in India.
In the Co-Win App, one can enter details like the name, address, mobile number and identity card of the person to get the vaccine. However, the government has not yet launched the mobile version of the Co-win app for the general public. The desktop version is the version designed for health workers. In this, the data of those health care workers who have been vaccinated in the first phase has been uploaded. Around 16,000 people in Meerut have been included in the category of a health care worker.
Claims, promises and photographs are all seen to be reassuring but the ground reality is just a little far from the claims. The boom seen in cities has not reached the health care centres of the villages. If the villagers are not sure of getting a doctor in the hospital, how can they believe that the vaccine will reach them in villages?
Currently, three companies in India have applied to the DGCI i.e. Drug Controller for emergency approval of their vaccine. It is expected that soon after approval, vaccination will start in India by early 2021. Along with the preparations of the government and administration, the primary health centres of the villages also need a little speed, as the country is eagerly waiting for the vaccine.
Rumour and truth: Bharat Biotech and ICMR’s indigenous coronavirus vaccine trial being made in India is in the third phase and researchers are having trouble finding volunteers in the third phase. People have become apprehensive about the vaccine after some side effects were reported during the trial of the corona vaccine.
Some such messages are also going viral on social media, which are going to create fear about the vaccine. You should also know the misconceptions and truths spread about this vaccine.
1. The myth is that the vaccine can alter your body’s DNA while the truth is that no such vaccine has been produced yet, which affects DNA.
2. The rumour is that once a vaccine is given, a lifetime of immunity can be found. That is, once the vaccine is administered, there will be no COVID-19 infection again. The truth is that whether the vaccine will give immunity for one year, of five years or lifetime, it is difficult to say that now.
3. There is also a belief that a single dose of vaccine is enough, even if you do not wear a mask after that. It is only rumoured, two doses of the vaccine will be applied at a difference of 21 and 28 days and even then it is necessary to use a mask. It is also important to take care of the distance of two yards and cleanliness.
4. The myth is that the side effects of the vaccine are worse than the side effects of the COVID-19. However, apart from mild fever and mild pain at the place of injection, no problems have been found so far.
Corona Vaccination-Information: If you have coronavirus or have symptoms such as fever, cough, you will not be vaccinated until you are fully recovered. Vaccination is not mandatory. If you do not want to get vaccinated, you will not be forcibly injected. Anti-body protection against vaccine will begin to form only two weeks after the second dose.
Live TV










